Disposable Non Sterile Surgical Face Mask
-
Price:
Negotiable
- minimum:
- Total supply:
-
Delivery term:
The date of payment from buyers deliver within days
-
seat:
Jiangsu
-
Validity to:
Long-term effective
-
Last update:
2022-01-15 05:17
-
Browse the number:
451
 Company Profile
Company Profile

By certification [File Integrity]
Contact:youlifu(Mr.)
Email:
Telephone:
Phone:
Area:Jiangsu
Address:Jiangsu
Website:http://www.ulfbiotech.com/ http://youlifu.hfyt168.com/
Product Details
Features:
Product Name |
disposable non sterile surgical face mask |
Brand |
ULF |
Certificate |
Doc, Class I EN 14683:2019+AC:2019 test report ISO 13484 |
TYPE |
IIR |
Specification |
For Adult 17.5*9.5cm |
Main material |
melt-brown fabric 33%, nonwoven fabric 67% |
Application |
General medical environment, daily prevention |
Package |
50PCS/box |
Description:
Our company has been listing in the whitelist of CCCM(No 1041), obtain certificates for European CE Mark registration.
We have established our own labs, have the most important test equipment, such as BFE、PFE test equipment, bacterial incubator, burn-in equipment and pressure sterilizer and so on.
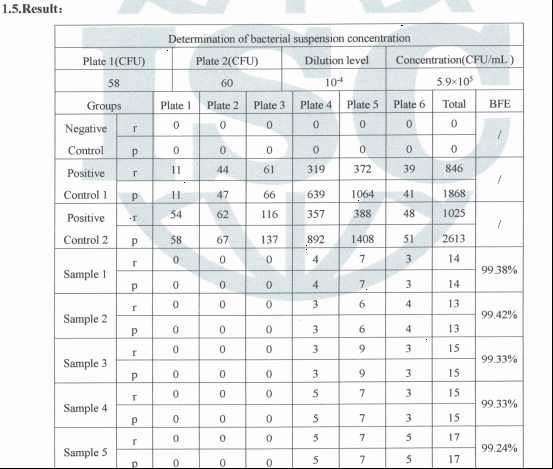
Meet type IIR requirement, BFE≥98%, Splash Resistance: 16 Kpa (120 mmHg).
Main materials: Nonwovens account for 67%, melt blown fabrics account for 33%.
Mask is suitable for wearing in general medical environment, blocking the exhalation of pollutants from mouth and nose.
Product Performance:
1. Passed EN14683-2019 test report.
2. The bacterial filtration efficiency of the mask shall not be less than 98%.
3. The ventilation resistance of air flow exchange on both sides of the mask shall not be greater than 49pa / cm2.
4. The product is not sterile.
Test report: EN 14683:2019+AC:2019


Workshop:



Lab pictures




http://www.ulfbiotech.com/
- Forklifts & Parts
- Gas Scooter
- UTV
- Bicycle Parts
- Other Bicycle Parts
- Bicycle Seat & Saddle
- Bicycle Pedal
- Bicycle Frame
- Bicycle Crank & Chainwheel
- Bicycle Wheel
- Bicycle Light
- Bicycle Hubs
- Bicycle Brake
- Bicycle Bell
- Bicycle Fork
- Bicycle Chain
- Bicycle Cassette & Freewheel
- Bicycle Handlebar
- Bicycle Stem
- Bicycle Handlebar Grips
- Bicycle Derailleur
- Bicycle Spoke
- Bicycle Seat Post
- Bike
- Electric Vehicle & Parts
- Barrow, Trolley & Cart
- Cargo & Storage
- Tractor
- Trailer
- Aviation
- Boats & Ships
- Container & Fittings
- Marine Parts
- Roadway Facility
- Elevator & Funicular Car
- Train & Railway
- Other Transportation Equipment


